|
Clinical Utility of Detection of Minimal Residual Disease by Flow Cytometry
In recent years several important prospective clinical studies have demonstrated that a slow response to induction chemotherapy and even persistence of a very low-level of leukemic blasts post induction are strong predictors of inferior outcome for pediatric patients with Acute Lymphoblastic Leukemia (ALL)1-4. Flow cytometry-determined MRD levels as low as 0.01% post induction adversely affected patient outcomes with further significant decrements in overall and event free survival at higher levels1. Moreover the levels of minimal residual disease (MRD) post induction were superior to the initial risk stratification based on conventional parameters1,4. New clinical trials are now seeking to address if early intervention such as extending and/or dose intensification of induction may prevent adverse outcomes in those with MRD post induction. Given the demonstrated clinical utility, laboratories receiving significant numbers of samples from patients with ALL should consider offering MRD testing.
MRD detection platforms
When applied properly either flow cytometry or molecular testing via polymerase chain reaction provide sufficient sensitivity of at least 1 leukemic blast in 10,000 cells in peripheral blood or bone marrow.
Molecular studies are limited by the requirement of pre-defined targets such as recurrent translocations or in their absence creation of patient specific primers or probes for T or B cell receptor rearrangements unique to the abnormal clone. In addition, neither of these PCR strategies is universally applicable due to lack of recurrent translocations in many ALL samples and presence of a subset of samples without demonstrable B or T cell receptor rearrangements. They also require that diagnostic material is available to define the PCR targets.
Flow cytometry, enjoys comparable sensitivity to PCR and is nearly universally applicable for MRD detection requiring presence of deviation from a normal pattern of lymphoid maturation rather then a priory-defined abnormalities. On the other hand flow cytometry requires significant technical expertise from both the laboratory staff and interpreting pathologist in order to avoid erroneous results. In this brief note we will cover some of the diagnostic and technical challenges in ALL MRD detection.
Technical Requirements for ALL MRD detection
In order to achieve the desired sensitivity of at least 1 leukemic blast in 10,000 cells that was found significant in clinical trials it is desirable to acquire at least 500,000 cells. Acquisition of this many cells allows for confident identification of clusters of at least fifty events at the lowest sensitivity threshold. It should be noted that a cluster containing as few as ten events with a clearly aberrant immunophenotype may be sufficient for confident identification. Identification of such small cell clusters requires a “clean” assay with low background. Various factors including non-specific antibody binding, improperly titered antibodies, inclusion of irrelevant events in blast analysis (dead cells, granulocytes, etc.), less then optimally cleaned fluidic system and carryover from prior cases can make for a suboptimal assay that can compromise specificity and sensitivity. Routine acquisition of a large number of events may only be practical on more modern high-speed digital acquisition flow cytometers. Such cytometers also frequently offer extended fluorescence detector arrays of 6 or more “colors”. The extended range of possible antibody combinations increases complexity of initial assay setup and places a premium on proper target antigen selection and fluorophore/antibody combination. Coverage of setup of multicolor assays is beyond the scope of this article and the reader is referred to many excellent resources covering this topic including the listed citations5,6. The reward for the increased amount of work during initial validation is an assay that allows greater confidence in the identification of abnormal events and is likely more sensitive due to the higher dimensionality of analysis. Detection of more antigens in a single tube also reduces the number of tubes needed for analysis saving time and reducing total number of cells needed to analyze a single case.
Interpretive aspects of ALL MRD detection
For initial diagnosis of leukemia it is frequently sufficient to simply enumerate blasts and assign lineage to the abnormal population. In this case demonstration of an expanded abnormal immature population using a marker of immaturity and two or more lineage markers may be all that is required for ALL diagnosis in the proper clinical context. Demonstration of aberrancy is frequently not necessary for diagnosis. However, extensive immunophenotyping using a combination of markers suitable for MRD detection is nonetheless preferable to establish a baseline for future evaluation.
Compared to initial diagnosis of ALL, MRD detection poses a set of unique challenges. MRD detection in B-ALL, particularly in later post-induction time points (post day 21), requires detection and enumeration of leukemic blasts in the context of normal, often regenerative, marrow that may contain numerous normal B-cell precursors. Demonstration of aberrancy in this context requires thorough understanding of the normal maturation pattern (Figure 1). Normal lymphoid maturation proceeds along a well-defined pattern of acquisition and loss of antigens7. In contrast, abnormal maturation may be loosely defined as deviation from the normal maturation pattern. Deviations may be due to changes in intensity of antigen expression, and maturation arrest, as well as gains and losses of antigen expression. As such, immunophenotypic aberrancy is best studied in the context of antigens that demonstrate maturation-associated changes.
Prior to the robust marrow regeneration that usually occurs following day 21 after initiation of induction a simpler approach using CD19 along with CD10 and CD34 has been proven to yield satisfactory results because hematogones are not expected to be present8. It should be noted that this approach is not applicable to B-ALL cases that lack expression of CD10 and CD34 at diagnosis such as a subset of B-ALL with 11q (MLL) abnormalities frequent in infants with ALL.
In the case of T-ALL, MRD detection in the blood or bone marrow is theoretically easier since most stages of normal T lymphocyte maturation occur in the thymus. Therefore the presence of an immature T cell subset in blood or bone marrow should be considered aberrant. Difficulty in T-ALL MRD detection largely stems from the loss of markers of immaturity on the abnormal blast population following induction chemotherapy. A recent study demonstrated that less then 60% of cases of T-ALL have markers of immaturity preserved on abnormal blasts following induction9. Moreover in many cases where partial preservation occurred, the antigens could not be used for enumeration due to the partial loss on the majority of blasts. In the absence of classical immaturity-associated antigen preservation it becomes critical to be able to discern abnormal blasts using other lineage-specific antigen aberrancies (Figure 2).
Immunophenotypic shifts in ALL
As already alluded to in the case of T-ALL, the immunophenotype of ALL blasts is fluid and may show significant changes in the context of induction chemotherapy. A trend toward a more mature phenotype as defined by the loss of immaturity-associated antigens is well demonstrated in both B and T-ALL9-11. In B-ALL losses of CD34 and CD10 are well demonstrated and are likely due to high dose corticosteroids10,11. In T-ALL CD99, TdT, CD10 and CD34 are not stably expressed following chemotherapy. Significant loss of these antigens on leukemic blasts can be seen as early as day 8 post induction. It is therefore not enough to merely look for blasts with a phenotype identical to that seen at presentation or to rely exclusively on immaturity associated antigens.
Robust approaches to MRD detection rely on casting a wide enough net to capture aberrant immunophenotypes even in the context of significant immunophenotypic shifts in immaturity-associated antigens. For instance in T-ALL expression of CD2, CD3, CD4, CD5, CD7, CD8 and CD45 remains relatively stable9. In addition, a combination of surface and cytoplasmic CD3 is of great utility in many cases. Care must be taken to exclude NK cell populations expressing CD16 and/or CD56; these cells often have variable expression of some T cell antigens and may mimic abnormal populations. In the case of B-ALL deviations from normal maturation patterns of expression of CD45, CD10, CD34, CD38, CD20, CD58 and light chain expression on CD19 positive B cell precursors are usually sufficient to detect abnormal populations even in the context of loss of one or more immaturity associated antigens.
Novel approaches to the discovery of antigens and antigenic combinations that assist in MRD evaluation and prediction of prognosis
Recent years have seen expansion of whole transcriptome analysis studies that have provided a great platform for non-biased discovery of leukemia-associated targets that may enhance MRD detection. These studies have already yielded such antigens as CD58 and CD49f that have been shown in clinical studies to be of value in B-ALL MRD detection12, 13. Other antigens will likely follow.
Whole genome expression analysis has also contributed to identification of subsets of ALL with particularly poor outcomes. A recent study in T-ALL has demonstrated that an early T cell precursor (ETP) immunophenotype identified a cohort with a very poor prognosis. Patients with T-ALL blasts demonstrating lack of expression of CD5, CD1a and CD8 in the context of aberrant myeloid or stem cell associated antigen expression had a very poor outcome and demonstrated high levels of MRD compared to cases with a more mature T-cell immunophenotype. It is likely that identification of this cohort of patients will become mainstay for the diagnostic evaluation of T-ALL by flow cytometry.
Challenges posed by ambiguous lineage leukemia and lymphoid blast crisis of CML
When evaluating for ALL it helps to be aware of subsets of cases that demonstrate cross-lineage differentiation that is best described as mixed phenotype leukemia rather then either ALL or acute myeloid leukemia (AML). These cases may demonstrate recurrence or MRD that is strongly biased or exclusive to a single lineage. Highlighting the complexity of leukemic differentiation are cases that do not initially meet criteria for leukemia of ambiguous lineage that nevertheless recur with a different differentiation pattern e.g. lymphoid blast recurrence of cases that met criteria for AML and vice versa. Lymphoid blast crisis of CML should also be kept in mind when evaluating cases presenting with an unusual confluence of findings e.g. a CML-like picture but with increased lymphoid blasts. These cases may well show recurrence with a different lineage.
Figure 1: B-ALL MRD in a background of normal B cell maturation

Figure 1:
Flow cytometric immunophenotyping performed on bone marrow using the following fluorochrome/antibody combination:
The top left plot shows all viable cells and subsequent plots displays B-cells identified through staining with CD19. Less mature B-cells are shown in aqua and more mature B-cells in blue. Normal B cell maturation proceeds along a well-defined pattern of gains and losses of antigens (demonstrated with red arrows). Early B cell precursors demonstrate intermediate expression of CD45 and low side scatter, bright expression of CD10 and CD38 and also express CD34. As the B cells mature they become slightly dimmer for CD10 and loose CD34 while gaining CD45 and beginning to acquire CD20. CD38 remains bright until mature levels of CD20 are acquired and CD10 is lost (shown in blue). CD20 acquisition parallels acquisition of surface light chains (not shown).
In contrast abnormal blasts (shown in red and emphasized) can demonstrate a variety of antigenic aberrancies that distinguish them from normally maturing B cell precursors. In this case the abnormal cells showed increased side scatter, dimmer CD45, brighter CD34 and CD38 and brighter CD58. This immunophenotype is very common in B-ALL.
Figure 2: A possible approach to detect T-ALL MRD that does not rely on markers of immaturity
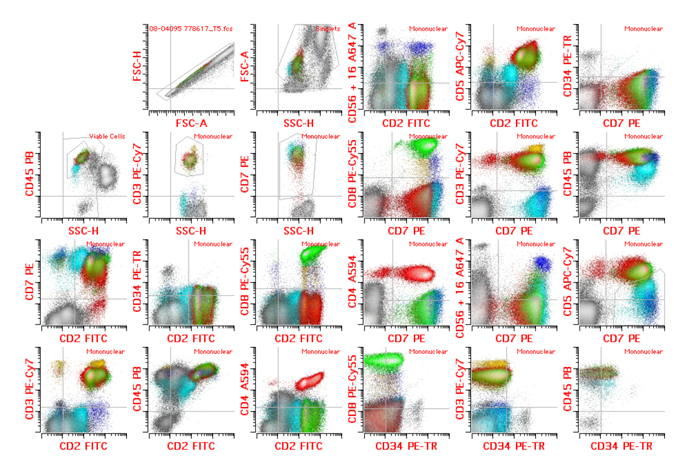
Figure 2:
Flow cytometric immunophenotyping performed on bone marrow using the following fluorochrome/antibody combination (CD2 FITC, CD7 PE, CD34-PETR, CD8 PE-CY5.5, CD3-PE-CY7, CD4 A594, CD56/CD16 A647, CD5 APC-CY7, CD45 PB).
Sequential gating to identify population of interest was applied as follows:
Doublet exclusion (excluding coincident events that can show compound immunophenotype) resulting in “Singlet” gating was followed by exclusion of debris, red cells and platelet clumps (forward vs. side scatter)- “Viable cell” gating. Granulocytes were excluded based on side scatter and CD45 resulting in “Mononuclear” cell gate containing lymphocytes, monocytes and blasts. Non-T cells (grey) were further excluded via lack of CD7. Normal CD8 positive T cells are shown in green. Normal CD4 positive T cells are shown in red. Gamma-delta T cells (bright CD3 without CD4 or significant CD8) are shown in yellow. NK cells (CD3 and CD5 negative having expression of CD16 or CD56) are shown in blue.
T-ALL MRD could be determined in the absence of immaturity-associated antigens. The case shows T-ALL MRD (aqua) with complete lack of TdT and CD99 at the level of mature T cells post induction (not shown). The blasts also lack CD34. Note that the abnormal population can still be detected and enumerated using lineage specific antigens. The abnormal blasts from the bone marrow showed dim CD45 with low side scatter as well as dim expression of T-lineage antigens CD2 and CD5 without surface CD3. The blasts also lacked expression of CD16, CD56, CD4 or CD8 while expressing bright CD7. This phenotype was similar to the patient’s blasts at presentation with the exception of previously bright expression of TdT and CD99.
Acknowledgements:
The author gratefully acknowledges Dr Sindhu Cherian for providing the B-ALL MRD images.
Mikhail Roshal, MD, PhD
Weill-Cornell Medical College, New York, NY
mir9079@med.cornell.edu
References:
1 Borowitz, M. J. et al. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children's Oncology Group study. Blood 111, 5477-5485
2 Schultz, K. R. et al. Risk- and response-based classification of childhood B-precursor acute lymphoblastic leukemia: a combined analysis of prognostic markers from the Pediatric Oncology Group (POG) and Children's Cancer Group (CCG). Blood 109, 926-935
3 Patel, B. et al. Minimal residual disease is a significant predictor of treatment failure in non T-lineage adult acute lymphoblastic leukaemia: final results of the international trial UKALL XII/ECOG2993. Br J Haematol 148, 80-89
4 Conter, V. et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood 115, 3206-3214
5 Wood, B. L. Ten-color immunophenotyping of hematopoietic cells. Curr Protoc Cytom Chapter 6, Unit6 21 (2005).
6 Wood, B. 9-color and 10-color flow cytometry in the clinical laboratory. Arch Pathol Lab Med 130, 680-690 (2006).
7 Lucio, P. et al. Flow cytometric analysis of normal B cell differentiation: a frame of reference for the detection of minimal residual disease in precursor-B-ALL. Leukemia 13, 419-427 (1999).
8 Coustan-Smith, E. et al. A simplified flow cytometric assay identifies children with acute lymphoblastic leukemia who have a superior clinical outcome. Blood 108, 97-102, 2006.
9 Roshal, M., Fromm, J. R., Winter, S., Dunsmore, K. & Wood, B. L. Immaturity associated antigens are lost during induction for T cell lymphoblastic leukemia: implications for minimal residual disease detection. Cytometry B Clin Cytom 78, 139-146 (2010).
10 Dworzak, M. N. et al. Modulation of antigen expression in B-cell precursor acute lymphoblastic leukemia during induction therapy is partly transient: evidence for a drug-induced regulatory phenomenon. Results of the AIEOP-BFM-ALL-FLOW-MRD-Study Group. Cytometry B Clin Cytom 78, 147-153, (2010).
11 Gaipa, G. et al. Drug-induced immunophenotypic modulation in childhood ALL: implications for minimal residual disease detection. Leukemia 19, 49-56 (2005).
12 Chen, J. S. et al. Identification of novel markers for monitoring minimal residual disease in acute lymphoblastic leukemia. Blood 97, 2115-2120 (2001).
13 DiGiuseppe, J. A., Fuller, S. G. & Borowitz, M. J. Overexpression of CD49f in precursor B-cell acute lymphoblastic leukemia: potential usefulness in minimal residual disease detection. Cytometry B Clin Cytom 76, 150-155 (2009).
|

