Ask an Expert
Back to Categories
What causes non-specific antibody binding and how can it be prevented?
Non-specific antibody binding occurs when an antibody binds to a cell that does not have an epitope specifically for that antibody. There are several reasons for non-specific antibody binding.
The most common cause is an excess of antibody. When antibody concentrations are too high, the antibody will bind to much lower affinity targets. This problem can be resolved by optimizing antibody concentration using a titration study. This will optimize signal-to-background staining.
Another reason for non-specific binding is the binding of antibody to Fc receptors, which are antibody-binding proteins found on the surface of immune cells, such as neutrophils, monocytes, macrophages, B-cells, natural killer cells, and some T-cell subsets. Fc regions of many antibodies readily bind to the Fc-receptors at different levels. Most commercially available antibodies contain Fc segments. To eliminate this problem, one can use an Fc blocking reagent, which contains a recombinant protein derived from immunoglobulin that will bind to the Fc-receptors and minimize the non-specific binding. Some vendors include the Fc blocking reagent in their antibody reagents, whereas some others sell it separately. Another way to reduce surface proteins is to trigger the process of endocytosis by incubating the specimen at 37°C for 30 minutes prior to antibody addition.
Non-viable cells can also be a source of cell clumping and non-specific binding. Dead cells are sticky, due in part to DNA exposed by their damaged cellular membrane. Non-viable cells can be excluded using a DNA binding dye (viability dye) such as 7-amino actinomycin-D (7-AAD) or propidium iodine (PI) included in the same tube with other antibodies. This approach is required for some assays, such as CD34+ stem cell enumeration (see CAP checklist FLO.30564). If a viability dye is not included in the same tube, a viability gate in the forward/side scatter dot plot can also help to separate viable and non-viable cells (see example below).
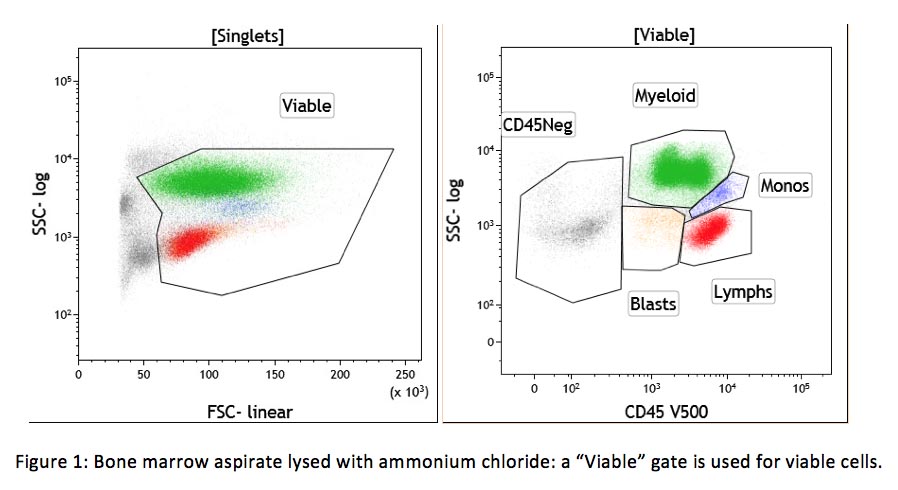
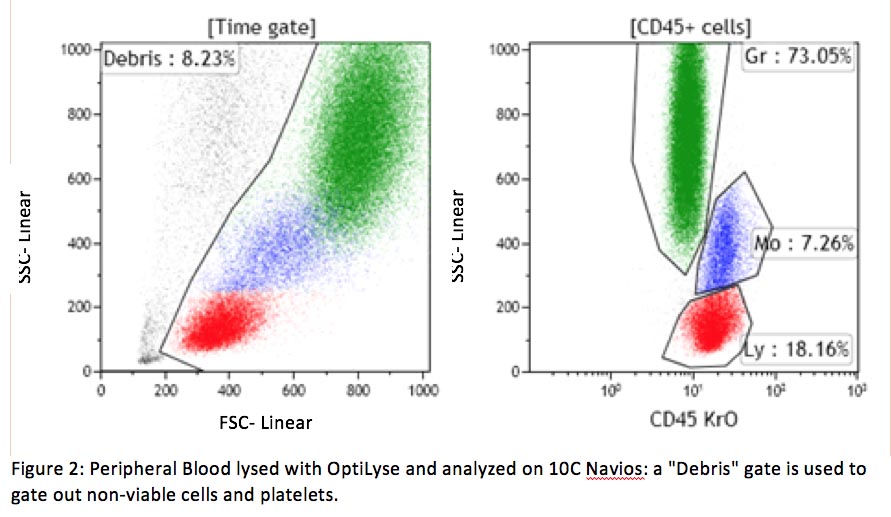
A lack of protein in the washing and staining solutions also contributes to the non-specific binding. All cells adhere to protein at various levels. If there is a lack of protein in the washing and staining solutions, antibodies (which are protein) will non-specifically bind to cells and cause high background fluorescence. This problem can easily be fixed by including bovine serum albumin (BSA) or fetal bovine serum (FBS) in these solutions.
Artifactual interactions between antibodies can also happen, especially with mouse IgG2 antibodies. This interaction is mediated by the plasma complement protein C1q. To prevent this unwanted association, one can avoid the use of IgG2 class antibodies or remove plasma prior to antibody addition. Plasma can be removed by repeated washing or prelysis of samples with NH4Cl followed by a single phosphate-buffered saline (PBS) wash.
Reference:
- Capel PJ, van de Winkel JG, van den Herik-Oudijk IE, Verbeek JS. Heterogeneity of human IgG Fc receptors. Immunomethods 1994; 4:25-34.
- Hulspas R, O'Gorman MRG, Wood BL, Gratama JW, Sutherland DR. Considerations for the control of background fluorescence in clinical flow cytometry. Cytometry Part B 2009; 76B: 355-364.
- Wood BL, Levin GR. Interactions Between Mouse IgG2 Antibodies Are Common and Mediated by Plasma C1q. Cytometry B Clin Cytom. 2006 Sep 15; 70(5):321-8.
Author: Kim Le
